MUC2 (mucin 2, oligomeric mucus/gel-forming)
2009-11-01 Suguru Yonezawa , Norishige Yamada , Seiya Yokoyama , Sho Kitamoto , Michiyo Higashi , Masamichi Goto AffiliationIdentity
HGNC
LOCATION
11p15.5
LOCUSID
ALIAS
MLP,MUC-2,SMUC
FUSION GENES
DNA/RNA
Note
MUC2 gene is the first human secretory mucin gene to be cloned and fully sequenced (Gum et al., 1994).
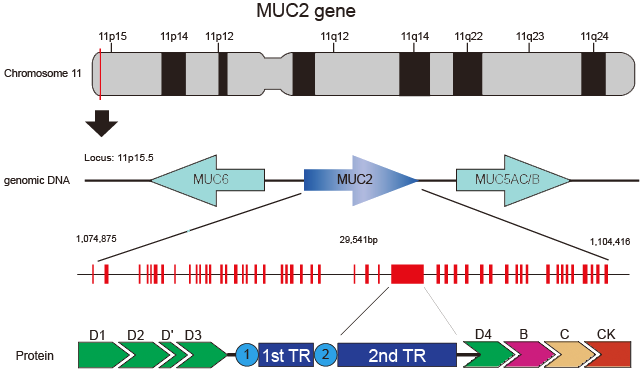
MUC2 is located at the telomeric end of about 400kb gene cluster on chromosome 11p15.5 and oriented from centromere to telomere and clustered with another secretory mucin genes such as MUC6 and MUC5AC/B. These genes show sequence homology in their non-tandem repeat domains and are thought to have arisen by duplication from a common ancestor. MUC2 is composed of 49 exons, exon 30 being the largest one and coding for the tandem repeats region. The array indicate the direction of transcription (not to scale).
Description
MUC2 is located within a 29-kb DNA fragment between MUC6, MUC5AC and MUCB on chromosome 11 in the region p15.5 (Griffiths et al., 1990; Desseyn et al., 1998; Rousseau et al., 2004), and cDNA is approximately 15.5 kb long. Genes that share some sequence homology are believed to have arisen by duplication from a common ancestor.
Transcription
The MUC2 promoter contains a TATA box, and includes binding sequence motifs for transcription factors such as AP2, SP1 and CDX2 (Griffiths et al., 1990; Toribara et al., 1991; Gum et al., 1994). MUC2 expression has been reported to be regulated by the Sp family (Nogami et al., 1997; Aslam et al., 2001). SP1 activates transcription synergistically both close to and far from the transcription start site in an enhancer-like manner. In several human cancer cell lines, p53 has also been indicated to regulate MUC2 transactivation by binding to two of its sites (-1131/-1100 and -676/-650) (Ookawa et al., 2002). Moreover, NF-kB and CDX2 have been reported to up-regulate MUC2 transcription (Yamamoto et al., 2003; Ikeda et al., 2007). Methylation of cytosine residues at CpG dinucleotides is an important epigenetic change that has been linked to transcriptional repression and regulation of chromatin structure (Holliday et al., 1975; Meehan et al., 2001; Recillas-Targa et al., 2002). Increased methylation of promoter region causes suppression of MUC2 gene (Hanski et al., 1997b; Riede et al., 1998). Hamada et al. have determined the detailed methylation status of a wide area of the MUC2 promoter region in pancreatic cancer cell lines, and suggested that methylation of certain CpG sites may play a particularly important role in the regulation of MUC2 transcription (Hamada et al., 2005). A CDX2 binding site is present downstream of the MUC2 promoter, and Yamamoto et al. have reported that the homeodomain protein CDX2 interacts with the MUC2-WT cis element (bases -201 to -162) in the MUC2 promoter and that ectopic expression of the CDX2 protein activates transcription of MUC2 (Yamamoto et al., 2003). In bisulfite genomic sequencing study, CDX2-binding CpG site showed 95% methylation in PANC1 (MUC2-negative) and 50% methylation in BxPC3 (MUC2-positive) (Hamada et al., 2005). Thus, MUC2 expression might be regulated by epigenetic changes in the promoter region containing the CDX2 binding site. In addition to DNA methylation, H3-K9 methylation is closely related to DNA methylation and acts as an epigenetic marker for silencing of the MUC2 gene (Yamada et al., 2006; Vincent et al., 2007).
Proteins
Note
MUC2 is one of the human gel-forming secreted mucins, and has a unique core peptide composed of 23 amino acids. The N-terminal and C-terminal regions of the MUC2 apomucin are very similar to von Willebrand factor (vWF). The N-terminal region is composed of disulfide-rich D-domains (D1, D2, D and D3) which is seen in vWF. The C-terminal region has also domains seen in vWF (D4, B, C and CK). In the central part, there are the first Ser/Thr/Pro rich 21 repetitive region showing an irregular amino acid motif, and the second regular tandemly repeated motifs of 23 amino acids (PTTTPITTTTTVTPTPTPTGTQT) which show variable number of tandem repeat from 51 to 115. CYS domain are present between the N-terminal region and the first Ser/Thr/Pro rich 21 repetitive region, and also present between the first Ser/Thr/Pro rich repetitive region and the second 23 amino acids repetitive region.
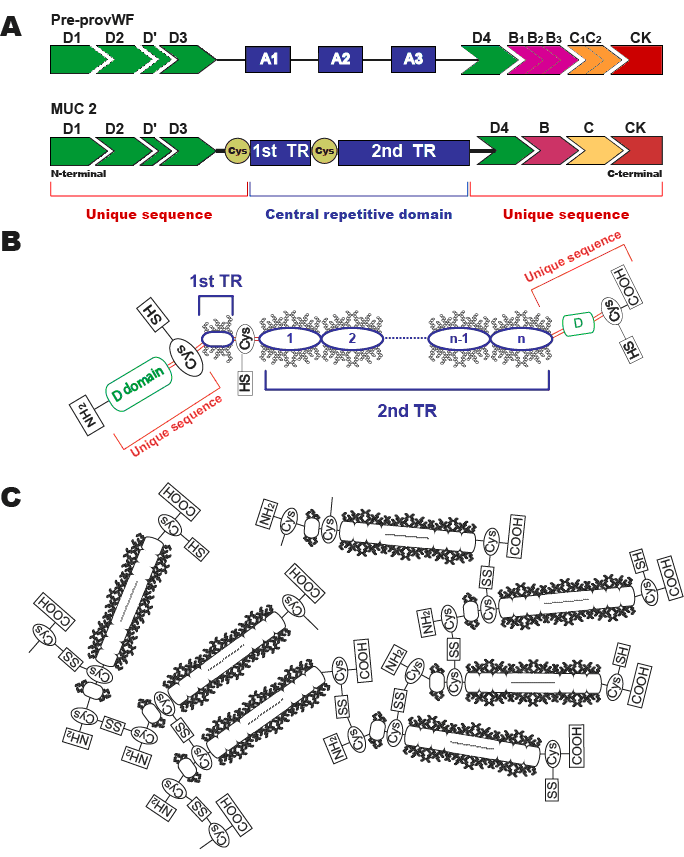
A: Schematic diagram of vWF (upper) and MUC2 mucin core protein (lower). N-terminal and C-terminal regions of MUC2 are highly similar to those of vWF, in its D, B, C and CK domains.
B: Illustration of the structure of MUC2 mucin monomer. The 1st tandem repeat (TR) region is Ser, Thr and Pro rich, and made of 21 repetitions of an irregular amino acid motif. The 2nd TR region show variable number of tandem repeat (VNTR) from 51 to 115 of TR composed of 23 amino acids.
C: The central repetitive domain is rigid owing to the heavy glycosylation. On the other hand, the unique sequences contain numerous cysteine residues that link monomers to oligomers via disulfide bonds.
Description
The central repetitive domains are rigid owing to heavy glycosylation, whereas the unique sequences of N-terminal and C-terminal regions sparsely glycosylated and protease-sensitive. In addition, the unique sequences have numerous Cys residues that link monomers to oligomers by disulfide bonds.
Expression
In normal physiological situation, MUC2 shows unique expression profiles in the limited organs and tissues such as intestine, bronchus and salivary gland.
In the normal human adult intestinal system, MUC2 expression is observed in the perinuclear areas of the goblet cells in the villi and crypts of the small intestine, and in the crypts of the colon and rectum, but not in the surface absorptive cells. MUC2 mRNA expression is also demonstrated at the perinuclear areas of those goblet cells (Chang et al., 1994; Buisine et al., 1998). Precise in situ hybridization (ISH) and immunohistochemistry (IHC) studies in electron microscopic level study demonstrated that MUC2 mRNA and MUC2 apomucin are located predominantly in the supranuclear and the perinuclear rough endoplasmic reticulum (RER), but not in the Golgi apparatus, in the goblet cells (Wang et al., 2001).
In ISH study in human embryonic and fetal tissues, in small intestine, MUC2 mRNA was detected between the primordial villi at 9 weeks after gestation, and was located mainly in immature crypts of Lieberkuhn after 10 weeks until 23 weeks. After that, MUC2 mRNA was expressed at the perinuclear regions of the goblet cells in villi and crypts. In the colon and rectum, MUC2 mRNA was detected at the perinulear regions of the goblet cells in crypts from 13 weeks of gestation (Buisine et al., 1998).
In the bronchial tissue, MUC2 was expressed below and around mucous secretory granules (RER and Golgi apparatus) in the bronchial surface epithelium and bronchial mucous glands (Jany et al., 1991).
Second-generation MAb to MUC2 (CCP58) detects weak expression in salivary glands in addition to the intense expression in the small and large intestine as described above glands (Xing et al., 1992).
In the normal human adult intestinal system, MUC2 expression is observed in the perinuclear areas of the goblet cells in the villi and crypts of the small intestine, and in the crypts of the colon and rectum, but not in the surface absorptive cells. MUC2 mRNA expression is also demonstrated at the perinuclear areas of those goblet cells (Chang et al., 1994; Buisine et al., 1998). Precise in situ hybridization (ISH) and immunohistochemistry (IHC) studies in electron microscopic level study demonstrated that MUC2 mRNA and MUC2 apomucin are located predominantly in the supranuclear and the perinuclear rough endoplasmic reticulum (RER), but not in the Golgi apparatus, in the goblet cells (Wang et al., 2001).
In ISH study in human embryonic and fetal tissues, in small intestine, MUC2 mRNA was detected between the primordial villi at 9 weeks after gestation, and was located mainly in immature crypts of Lieberkuhn after 10 weeks until 23 weeks. After that, MUC2 mRNA was expressed at the perinuclear regions of the goblet cells in villi and crypts. In the colon and rectum, MUC2 mRNA was detected at the perinulear regions of the goblet cells in crypts from 13 weeks of gestation (Buisine et al., 1998).
In the bronchial tissue, MUC2 was expressed below and around mucous secretory granules (RER and Golgi apparatus) in the bronchial surface epithelium and bronchial mucous glands (Jany et al., 1991).
Second-generation MAb to MUC2 (CCP58) detects weak expression in salivary glands in addition to the intense expression in the small and large intestine as described above glands (Xing et al., 1992).
Localisation
After the multimerization forming oligomers and O-glycosylation, MUC2 mucin is stored in secretory granules. MUC2 is localized in the supra- and peri-nuclear areas, which are RER (Wang et al., 2001), of the columnar epithelium with goblets in small and large intestine. MUC2 is also seen in the peribronchial glands.
Function
MUC2 mucin form gel layer which covers the intestinal mucosa, protect the mucosal surface, and plays a role of lubricant particularly at the large intestine. MUC2 also has a function as a tumor suppressor. MUC2 knockout mice frequently develop tumors in the small intestine, colon and rectum where MUC2 is predominantly expressed in the normal condition (Velcich et al., 2002). Alteration in protection and lubrication of the intestinal mucosal surface by the lack of MUC2 might induce an increase of bacterial flora carrying pro-carcinogenic effect, or might result in release of intestinal mucosa-derived factors which are normally depressed by MUC2 stimulating pro-carcinogenic factors, or might lead to destruction of a physical barrier of MUC2 to dietary carcinogens. Loss of MUC2 might compromise the signaling which contributes to the epithelial differentiation and proliferation through the contacts with membrane-bound mucins, or might alterate in the differentiation program of the intestinal mucosa resulting in the increased probability of tumor formation. High levels of MUC2 expression in indolent human pancreatobiliary neoplasms with a favorable prognosis (Osako et al., 1993; Yamashita et al., 1993; Yonezawa et al., 1997b; Yonezawa et al., 1997a; Yonezawa et al., 2008a) might be related with tumor suppressor activity by MUC2.
Homology
The human gel-forming mucins, MUC5AC, MUC5B and MUC6, whose genes are located on chromosome 11p15.5 like MUC2, show homology with MUC2. MUC19, whose gene is located on chromosome 12q12, also has homology with MUC2. N-terminal and C-terminal regions of MUC5AC and MUC5B are same as those of MUC2, and are highly similar to those of vWF, in their D, B, C and CK domains which are observed in vWF. N-terminal regions of MUC6 and MUC19 are also same as that of MUC2, but the C-terminal region of MUC6 has only CK domain, and that of MUC19 has C and CK domains.
Implicated in
Entity name
Pancreatic neoplasms
Disease
MUC2 shows unique expression profiles related with MUC1 as follows, in our serial IHC studies. Pancreatic ductal adenocarcinoma (PDAC) with aggressive biological behavior and poor outcome usually showed expression of MUC1 (pan-epithelial type membrane-associated mucin) but no expression of MUC2 (Osako et al., 1993). In contrast, intraductal papillary mucinous neoplasm (IPMN) with indolent biological behavior showed no expression of MUC1, and are divided into subtypes by MUC2 expression as well as the morphological appearances, i.e., IPMN-intestinal type with MUC2 expression and IPMN-gastric type without MUC2 expression (Yonezawa et al., 1999; Nakamura et al., 2002; Horinouchi et al., 2003). The IPMN-intestinal type shows sometimes malignant transformation with MUC1 expression in the cancerous tissue, resulting in poor outcome (Yonezawa et al., 1998; Nakamura et al., 2002). In contrast, the IPMN-gastric type shows rare malignant transformation (Nakamura et al., 2002; Horinouchi et al., 2003). MUC2 expression is very useful to differentiate the IPMN-intestinal type from precursor lesions of PDACs, pancreatic intraepithelial neoplasms (PanINs) which never express MUC2 (Nagata K et al., 2007; Yonezawa et al., 2008b; Yonezawa et al., 2009).
Entity name
Biliary neoplasms
Disease
Also in bile duct neoplasms, similar phenomena are observed in our series of IHC studies for mucin expression in various bile duct neoplasms have demonstrated that the expression of MUC1 is related to invasive proliferation of tumors and/or a poor outcome for patients. On the other hand, the expression of MUC2 is related to non-invasive proliferation of tumors and/or a favorable outcome for patients (Yamashita et al., 1993; Kitamura et al., 1996; Higashi et al., 1999; Tamada et al., 2002; Yonezawa et al., 2002; Shibahara et al., 2004; Yonezawa et al., 2008a; Yonezawa et al., 2008b; Yonezawa et al., 2009).
Entity name
Gastric carcinoma
Disease
Combined evaluation of MUC1 and MUC2 expression in gastric cancer demonstrated that the patients with gastric adenocarcinomas carrying MUC1+/MUC2- pattern showed the worst outcome, whereas those carrying MUC1-/MUC2+ pattern showed the best outcome (Utsunomiya et al., 1998).
Entity name
Colorectal neoplasm
Disease
In colorectal neoplasms, increased MUC1 expression and reduced MUC2 expression are related to adenomas with high grade atypia or malignant transformation (Ajioka et al., 1997; Li et al., 2001).
In gastrointestinal tract, MUC2 expression shows interesting contrasts with MUC5AC (gastric type secreted mucin). In the gastric mucosa with intestinal metaplasia, the area of intestinal metaplasia is MUC2 positive but MUC5AC negative, whereas the normal gastric mucosa is MUC2 negative but MUC5AC positive. Gastric and colorectal neoplasms are also classified as intestinal type (MUC2+/MUC5AC-), gastric type (MUC2-/MUC5AC+) and their mixed type, according to the mucin expression patterns (Yao et al., 2001; Tsukashita et al., 2001).
In gastrointestinal tract, MUC2 expression shows interesting contrasts with MUC5AC (gastric type secreted mucin). In the gastric mucosa with intestinal metaplasia, the area of intestinal metaplasia is MUC2 positive but MUC5AC negative, whereas the normal gastric mucosa is MUC2 negative but MUC5AC positive. Gastric and colorectal neoplasms are also classified as intestinal type (MUC2+/MUC5AC-), gastric type (MUC2-/MUC5AC+) and their mixed type, according to the mucin expression patterns (Yao et al., 2001; Tsukashita et al., 2001).
Entity name
Mucinous carcinomas of the colon, pancreas, breast and ovary
Disease
In ovarian tumors, colon adenocarcinomas metastatic to ovary show MUC2+/MUC5AC- pattern, whereas many of primary ovarian mucinous cystadenocarcinomas show MUC2+/MUC5AC+ pattern. From these findings, combined evaluation of MUC2 and MUC5AC may be useful in the distinction between them (Albarracin et al., 2000).
On the other view points, overexpression or ectopic expression of MUC2 is the common phenomenon to mucinous carcinomas of the colon, pancreas, breast and ovary, indicating a common genetic lesion associated with the mucinous tumor phenotype (Tashiro et al., 1994; Yonezawa et al., 1995; Hanski et al., 1997a; Matsukita et al., 2003).
On the other view points, overexpression or ectopic expression of MUC2 is the common phenomenon to mucinous carcinomas of the colon, pancreas, breast and ovary, indicating a common genetic lesion associated with the mucinous tumor phenotype (Tashiro et al., 1994; Yonezawa et al., 1995; Hanski et al., 1997a; Matsukita et al., 2003).
Entity name
Cystic fibrosis
Disease
In cystic fibrosis (CF), which is a genetic disorder associated with defects of cystic fibrosis transmembrane conductance regulator (CFTR) gene, and the plugging of the ducts of exocrine glands by viscid mucin is a major manifestation, MUC2 gene is expressed at 3 to 4 higher levels in CF nasal mucosa than in non-CF nasal tissue (Li et al., 1997).
Prognosis
Generally, expression of MUC2 is related to non-invasive proliferation of tumors and/or a favorable prognosis for the patients, whereas expression of MUC1 is related to invasive proliferation of tumors and/or a poor outcome for the patients (Osako et al., 1993; Yamashita et al., 1993; Kitamura et al., 1996; Yonezawa et al., 1997b; Yonezawa et al., 1997a; Utsunomiya et al., 1998; Higashi et al., 1999; Tamada et al., 2002; Yonezawa et al., 2008a). But, in indolent pancreatobiliary neoplasms such as IPMN-intestinal type or mucin-producing bile duct tumor (MPBT)-columnar type with MUC2 expression show sometimes invasive proliferation with aberrant MUC1 expression and show poorer prognosis compared with the other indolent panceratobiliary neoplasms such as IPMN-gastric type or MPBT-cuboidal type with no or low MUC2 expression (Nakamura et al., 2002; Horinouchi et al., 2003; Shibahara et al., 2004).
Article Bibliography
| Pubmed ID | Last Year | Title | Authors |
|---|---|---|---|
| 9215126 | 1997 | MUC1 and MUC2 mucins in flat and polypoid colorectal adenomas. | Ajioka Y et al |
| 10872659 | 2000 | Differential expression of MUC2 and MUC5AC mucin genes in primary ovarian and metastatic colonic carcinoma. | Albarracin CT et al |
| 11212251 | 2001 | The Sp family of transcription factors in the regulation of the human and mouse MUC2 gene promoters. | Aslam F et al |
| 9824580 | 1998 | Mucin gene expression in human embryonic and fetal intestine. | Buisine MP et al |
| 8020672 | 1994 | Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. | Chang SK et al |
| 9419229 | 1998 | Evolutionary history of the 11p15 human mucin gene family. | Desseyn JL et al |
| 1980995 | 1990 | Assignment of the polymorphic intestinal mucin gene (MUC2) to chromosome 11p15. | Griffiths B et al |
| 8300571 | 1994 | Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. | Gum JR Jr et al |
| 16112420 | 2005 | Mapping of the methylation pattern of the MUC2 promoter in pancreatic cancer cell lines, using bisulfite genomic sequencing. | Hamada T et al |
| 9306958 | 1997 | Overexpression or ectopic expression of MUC2 is the common property of mucinous carcinomas of the colon, pancreas, breast, and ovary. | Hanski C et al |
| 9426407 | 1997 | MUC2 gene suppression in human colorectal carcinomas and their metastases: in vitro evidence of the modulatory role of DNA methylation. | Hanski C et al |
| 10573510 | 1999 | Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. | Higashi M et al |
| 1111098 | 1975 | DNA modification mechanisms and gene activity during development. | Holliday R et al |
| 17417665 | 2007 | Interaction of Toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. | Ikeda H et al |
| 1985113 | 1991 | Human bronchus and intestine express the same mucin gene. | Jany BH et al |
| 8766528 | 1996 | Expression of mucin carbohydrates and core proteins in carcinomas of the ampulla of Vater: their relationship to prognosis. | Kitamura H et al |
| 11844051 | 2001 | Expression of MUC1 and MUC2 mucins and relationship with cell proliferative activity in human colorectal neoplasia. | Li A et al |
| 9155717 | 1997 | Localization and up-regulation of mucin (MUC2) gene expression in human nasal biopsies of patients with cystic fibrosis. | Li D et al |
| 12493022 | 2003 | Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: comparison with invasive ductal carcinoma. | Matsukita S et al |
| 11758457 | 2001 | DNA methylation and control of gene expression in vertebrate development. | Meehan RR et al |
| 17520199 | 2007 | Mucin expression profile in pancreatic cancer and the precursor lesions. | Nagata K et al |
| 12015744 | 2002 | New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. | Nakamura A et al |
| 9370281 | 1997 | Sp1 protein contributes to airway-specific rat MUC 2 mucin gene transcription. | Nogami H et al |
| 12374798 | 2002 | Transcriptional activation of the MUC2 gene by p53. | Ookawa K et al |
| 8384065 | 1993 | Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. | Osako M et al |
| 12459312 | 2002 | DNA methylation, chromatin boundaries, and mechanisms of genomic imprinting. | Recillas-Targa F et al |
| 14518264 | 1998 | [Increased methylation of promotor region suppresses expression of MUC2 gene in colon carcinoma cells]. | Riede E et al |
| 15081123 | 2004 | The complete genomic organization of the human MUC6 and MUC2 mucin genes. | Rousseau K et al |
| 15104295 | 2004 | Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. | Shibahara H et al |
| 12685548 | 2002 | Expression of MUC1 and MUC2 mucins in extrahepatic bile duct carcinomas: its relationship with tumor progression and prognosis. | Tamada S et al |
| 8163269 | 1994 | Immunohistochemical study of mucin carbohydrates and core proteins in human ovarian tumors. | Tashiro Y et al |
| 1885763 | 1991 | MUC-2 human small intestinal mucin gene structure. Repeated arrays and polymorphism. | Toribara NW et al |
| 11668493 | 2001 | MUC gene expression and histogenesis of adenocarcinoma of the stomach. | Tsukashita S et al |
| 9829723 | 1998 | Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. | Utsunomiya T et al |
| 11872843 | 2002 | Colorectal cancer in mice genetically deficient in the mucin Muc2. | Velcich A et al |
| 17471237 | 2007 | Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. | Vincent A et al |
| 11724906 | 2001 | Altered GalNAc-alpha-2,6-sialylation compartments for mucin-associated sialyl-Tn antigen in colorectal adenoma and adenocarcinoma. | Wang F et al |
| 1569603 | 1992 | Second-generation monoclonal antibodies to intestinal MUC2 peptide reactive with colon cancer. | Xing PX et al |
| 16721789 | 2006 | MUC2 expression is regulated by histone H3 modification and DNA methylation in pancreatic cancer. | Yamada N et al |
| 12559945 | 2003 | Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. | Yamamoto H et al |
| 8393843 | 1993 | Immunohistochemical study of mucin carbohydrates and core proteins in hepatolithiasis and cholangiocarcinoma. | Yamashita K et al |
| 11473726 | 2001 | Phenotypic expression of colorectal adenocarcinomas with reference to tumor development and biological behavior. | Yao T et al |
| 19787286 | 2010 | Significance of mucin expression in pancreatobiliary neoplasms. | Yonezawa S et al |
Other Information
Locus ID:
NCBI: 4583
MIM: 158370
HGNC: 7512
Ensembl: ENSG00000198788
Variants:
dbSNP: 4583
ClinVar: 4583
TCGA: ENSG00000198788
COSMIC: MUC2
RNA/Proteins
| Gene ID | Transcript ID | Uniprot |
|---|---|---|
| ENSG00000198788 | ENST00000616479 | Q9UMI9 |
Expression (GTEx)
Pathways
Protein levels (Protein atlas)
References
| Pubmed ID | Year | Title | Citations |
|---|---|---|---|
| 38500386 | 2024 | POFUT1 and PLAGL2 are characteristic markers of mucinous colorectal cancer associated with MUC2 expression. | 0 |
| 38500386 | 2024 | POFUT1 and PLAGL2 are characteristic markers of mucinous colorectal cancer associated with MUC2 expression. | 0 |
| 36367122 | 2023 | Evaluation of MUC1, MUC2, MUC5AC, and MUC6 Expression Differences in Lung Adenocarcinoma Subtypes by Using a Final Immunoreactivity Score (FIRS). | 0 |
| 37686224 | 2023 | An Increase in Mucin2 Expression Is Required for Colon Cancer Progression Mediated by L1. | 0 |
| 36367122 | 2023 | Evaluation of MUC1, MUC2, MUC5AC, and MUC6 Expression Differences in Lung Adenocarcinoma Subtypes by Using a Final Immunoreactivity Score (FIRS). | 0 |
| 37686224 | 2023 | An Increase in Mucin2 Expression Is Required for Colon Cancer Progression Mediated by L1. | 0 |
| 33515627 | 2022 | Next-generation sequencing analysis suggests varied multistep mutational pathogenesis for endocrine mucin-producing sweat gland carcinoma with comments on INSM1 and MUC2 suggesting a conjunctival origin. | 3 |
| 34086646 | 2022 | Utility of Insulinoma-Associated Protein 1 (INSM1) and Mucin 2 (MUC2) Immunohistochemistry in the Distinction of Endocrine Mucin-Producing Sweat Gland Carcinoma From Morphologic Mimics. | 3 |
| 34807735 | 2022 | Enterotoxigenic Escherichia coli Degrades the Host MUC2 Mucin Barrier To Facilitate Critical Pathogen-Enterocyte Interactions in Human Small Intestine. | 15 |
| 35757703 | 2022 | Endotoxins Induced ECM-Receptor Interaction Pathway Signal Effect on the Function of MUC2 in Caco2/HT29 Co-Culture Cells. | 6 |
| 33515627 | 2022 | Next-generation sequencing analysis suggests varied multistep mutational pathogenesis for endocrine mucin-producing sweat gland carcinoma with comments on INSM1 and MUC2 suggesting a conjunctival origin. | 3 |
| 34086646 | 2022 | Utility of Insulinoma-Associated Protein 1 (INSM1) and Mucin 2 (MUC2) Immunohistochemistry in the Distinction of Endocrine Mucin-Producing Sweat Gland Carcinoma From Morphologic Mimics. | 3 |
| 34807735 | 2022 | Enterotoxigenic Escherichia coli Degrades the Host MUC2 Mucin Barrier To Facilitate Critical Pathogen-Enterocyte Interactions in Human Small Intestine. | 15 |
| 35757703 | 2022 | Endotoxins Induced ECM-Receptor Interaction Pathway Signal Effect on the Function of MUC2 in Caco2/HT29 Co-Culture Cells. | 6 |
| 33559155 | 2021 | DOCK4 stimulates MUC2 production through its effect on goblet cell differentiation. | 12 |
Citation
Suguru Yonezawa ; Norishige Yamada ; Seiya Yokoyama ; Sho Kitamoto ; Michiyo Higashi ; Masamichi Goto
MUC2 (mucin 2, oligomeric mucus/gel-forming)
Atlas Genet Cytogenet Oncol Haematol. 2009-11-01
Online version: http://atlasgeneticsoncology.org/gene/41457/muc2-(mucin-2-oligomeric-mucus-gel-forming)
