PRND (Prion Protein 2 (Dublet))
2013-12-01 Gabriele Giachin , Giuseppe Legname AffiliationLaboratory of Prion Biology, Department of Neuroscience, Scuola Internazionale Superiore di Studi Avanzati (SISSA), via Bonomea 265, Trieste, Italy
Identity
HGNC
LOCATION
20p13
IMAGE

LEGEND
Schematic structural representation of the human PRN locus on chromosome 20p13 containing PRNP, PRND and the putative testis-specific prion protein (PRNT) genes.
LOCUSID
ALIAS
DOPPEL,DPL,PrPLP,dJ1068H6.4
FUSION GENES
DNA/RNA
Note
The Prnd gene was originally identified in mice during DNA sequencing of the cosmid clone isolated from the I/LnJ inbred mice strain (Lee et al., 1998). This gene was discovered in transgenic (Tg) mice where the Prnp gene was ablated (Prnp0/0 mice strains) resulting in a diseased phenotype characterized by loss of Purkinje cells in the cerebellum. Interestingly, Prnp deletion in these mouse lines resulted in the formation of a chimeric Prnd transcript under the control of the strong Prnp promoter. Thus, these studies have shown that only the ectopic expression of Dpl, rather than the absence of the Prnp gene, caused neurodegeneration (Li et al., 2000).
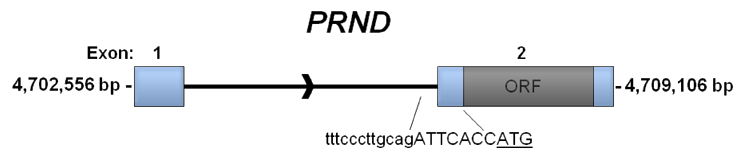
Schematic representation of the PRND gene. Exon 1 starts at 4705556 bp and ends at 4702615 bp. Exon 2 containing the Doppel open reading frame (ORF) starts at 4705187 bp and ends at 4709106 bp. The sequence surrounding the splice acceptor site is shown with intronic nucleotides in lower case, exonic nucleotides in capital letters and Met start codon ATG underlined.
Description
The PRND gene includes two exons separated by one intron. Exon 2 encodes for the Doppel protein.
Transcription
Prnd RNA transcription has been reported in different tissues of adult wild-type (WT) mice including testis, heart, spleen and skeletal muscle (Li et al., 2000). In neonatal mice up to 3 weeks, Prnd RNA has been detected in brain blood vessel endothelial cells (Li et al., 2000).
Pseudogene
Prnd pseudogenes have been identified in non-mammalian organisms as Anolis (lizard) and Xenopus (frog) (Harrison et al., 2010).
Proteins
Note
Doppel tertiary structure has a fold similar to that of the cellular prion protein, PrPC (encoded by PRNP or Prnp genes) although it shares approximately 25% of aminoacidic sequence identity with PrPC.
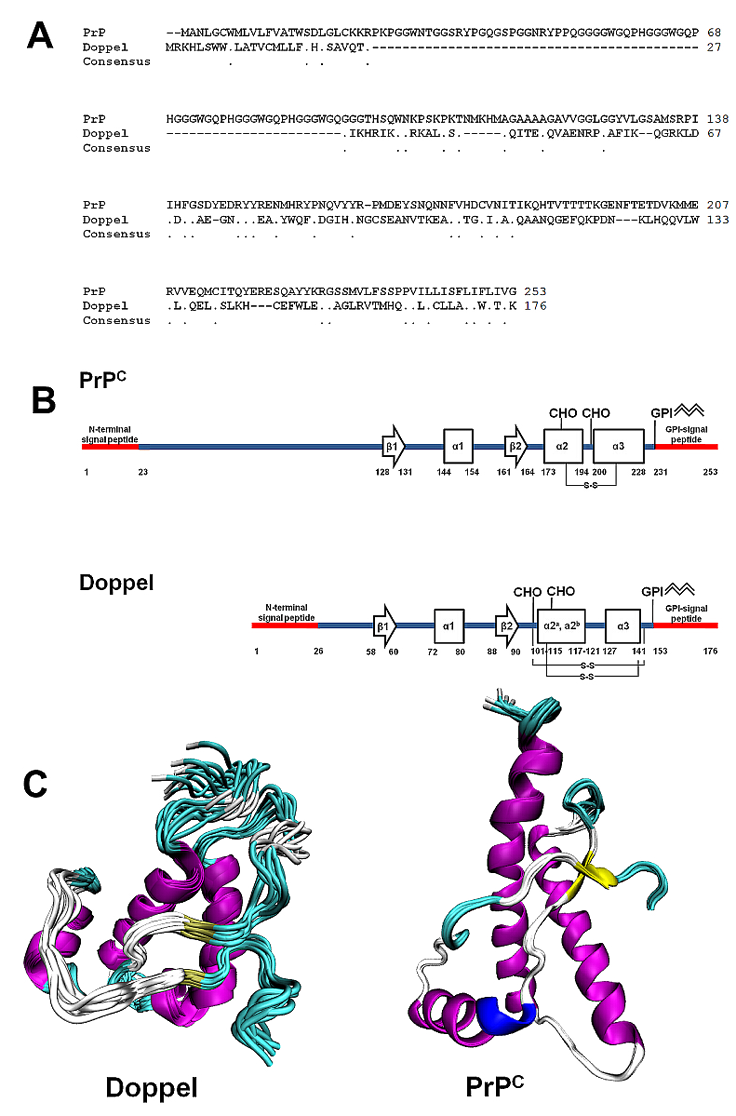
A) Primary sequence alignment between human PrPC (GenBank: BAG32277.1) and human Doppel (NCBI Reference Sequence: NP_036541.2). B) Secondary structure motives of human PrPC and Doppel. Highlighted: signal peptides, N-linked glycosylation sites (CHO), disulfide bridges (S-S) and Glycosylphosphatidylinisotol (GPI) anchor. C) Tertiary NMR structures of Doppel (pdb id 1LG4) and PrPC (2LSB).
Description
The immature form of human Doppel includes 176 residues with two N- and C-terminal signal peptides cleaved during protein maturation. The mature sequence includes 126 amino acids spanning from residues 27 to 152, with a molecular weight of approximately 14.5 kDa. Tryptic digestion and mass spectroscopy studies have identified two distinct disulfide bridges (Cys109-Cys143 and Cys95-Cys148) which strongly stabilize the Doppel folding (Baillod et al., 2013; Silverman et al., 2000; Whyte et al., 2003). PNGase F digestion and immunoblots have reported two N-linked glycosylation sites at codons 99 and 111. The GPI anchor targets the protein at the extracellular membrane. NMR structures of recombinant human and mouse Dopple have been solved (Luhrs et al., 2003; Mo et al., 2001). The NMR structures of the N-terminal murine and ovine signal peptides (residues 1-30) have also been determined (Papadopoulos et al., 2006). The human Doppel NMR structure features a short flexible N-terminal segment comprising residues 24-51 and a globular domain including four α-helices (α1: residues 72-80; α2a: residues 101-115; α2b: residues 117-121; α3: residues 127-141) and a short two-stranded anti-parallel β-sheet (β1: residues 58-60; β2: residues 88-90) (Luhrs et al., 2003).
Expression
Under physiological conditions Doppel is mostly expressed in testis and, in particular, in spermatozoa and Sertoli cells (Behrens et al., 2002; Peoch et al., 2002). Additionally, Doppel is expressed with PrPC in spleen cells, notably B lymphocytes, granulocytes and dendritic cells (Cordier-Dirikoc et al., 2008).
Localisation
Doppel is attached to the cell membrane through its GPI anchor (Silverman et al., 2000). A study has shown Doppel localization in detergent-resistant membranes or lipid rafts (Caputo et al., 2010).
Function
The Doppel expression in spermatozoa and Sertoli cells infers a role in spermatogenesis. Male Tg mice knock-out for Prnd were sterile, clearly indicating that Doppel plays a role in male reproduction as critical regulator of spermatogenesis and sperm-egg interaction (Behrens et al., 2002). Doppel may enhance in vitro ovine spermatozoa fertilizing ability (Pimenta et al., 2012). Doppel has been implicated in early testis differentiation (Kocer et al., 2007). The detection of Prnd mRNA in brain blood vessel endothelial cells might indicate a possible role in the development of brain blood vessels (Li et al., 2000). The observation that Doppel is expressed with PrPC in B lymphocytes, granulocytes and dendritic cells argues for a role in cell-cell interaction in the immunosystem (Cordier-Dirikoc et al., 2008). Several evidence showed that Doppel is able to coordinate in vitro the binding of copper ions with high affinity (Cereghetti et al., 2004; La Mendola et al., 2010; Qin et al., 2003).
Mutations
Note
Different polymorphic variants have been identified in PRND. The effect of polymorphisms in Doppel function and their implication in the diseases have not been fully clarified.
Germinal
S6I, S22P, T26P, H31R, P56L, F70L, L149S, T174M (Clark et al., 2003; Moore et al., 1999; Peoch et al., 2000; Schroder et al., 2001).
Implicated in
Entity name
Ectopic Doppel expression associated with Purkinje cell neurodegeneration in transgenic mouse models.
Note
Beside its role in male reproductive system, Doppel has attracted interest for its neurotoxic properties when ectopically expressed in the brain of Tg mice knock-out for the prion protein gene (Prnp0/0 mice). In these mice, denoted as Ngsk PrP-/-, the Doppel-encoding exon was expressed as chimeric mRNA due to the intergenic splicing taking place between Prnp and Prnd. As a result, Prnd became abnormally regulated under the control of Prnp promoter and ectopically expressed in the brain and, in particular, in neurons and glial cells (Li et al., 2000). Similar non-physiological Doppel expression was reported in other Tg mouse lines knock-out for Prnp such as Rcm0 and Zrch mice (Moore et al., 2001; Rossi et al., 2001). Doppel expression in the brain is neurotoxic and causes Purkinje cell degeneration in these mouse models. Doppel neurotoxicity is antagonized by the PrPC N-terminal domain (Atarashi et al., 2003; Yamaguchi et al., 2004). The neuroprotective PrPC role against ectopic Doppel expression has been reported also in human neuronal SH-SY5Y cells (Li et al., 2009) confirming the dominant-negative effects of the PrPC N-terminal region (Yoshikawa et al., 2008). The molecular mechanisms leading to Doppel-induced neurodegeneration in Purkinje and granular cells are still controversial. An earlier study has reported that the chimeric form of Doppel fused to a Fc domain binds specifically granule cells and causes neurodegeneration, raising the possibility that these specific cells expressed a still unidentified protein that mediates the Doppel-induced neurotoxicity (Legname et al., 2002). Oxidative stress may play a role in Doppel-induced neuronal death since NOS activity is induced by Doppel in vitro and in vivo (Cui et al., 2003; Wong et al., 2001). Two independent groups have reported that BAX contributes to Doppel-induced apoptosis (Didonna et al., 2012; Heitz et al., 2007) and that BCL-2 antagonizes Doppel neurotoxicity (Heitz et al., 2008). Another work has observed that ectopic Doppel expression in the brain elicits neurodegeneration through the binding of two metalloproteinase namely the alpha-1-inhibitor-3 (α1I3) and the alpha-2-macroglobin (α2M) (Benvegnu et al., 2009).
Entity name
Abnormal Doppel expression levels in human astrocytomas and other non-glial brain tumor specimens
Note
Doppel is aberrantly expressed in astrocytic tumors where it displays cytoplasmic, nuclear and lysosomal localization and molecular properties (i.e. altered glycosilation pattern) different from Doppel as normally expressed in testis (Azzalin et al., 2006; Azzalin et al., 2008; Comincini et al., 2006; Comincini et al., 2004; Comincini et al., 2007; Rognoni et al., 2010; Sbalchiero et al., 2008).
Article Bibliography
Other Information
Locus ID:
NCBI: 23627
MIM: 604263
HGNC: 15748
Ensembl: ENSG00000171864
Variants:
dbSNP: 23627
ClinVar: 23627
TCGA: ENSG00000171864
COSMIC: PRND
RNA/Proteins
Expression (GTEx)
Pathways
Protein levels (Protein atlas)
References
Citation
Gabriele Giachin ; Giuseppe Legname
PRND (Prion Protein 2 (Dublet))
Atlas Genet Cytogenet Oncol Haematol. 2013-12-01
Online version: http://atlasgeneticsoncology.org/gene/44172/prnd-(prion-protein-2-(dublet))
