Nervous system: Embryonal tumors: Neuroblastoma
2019-10-01 Caileigh Pudela , Skye Balyasny , Mark A. Applebaum Affiliation1.Department of Pediatrics, University of Chicago, Chicago, Illinois, mapplebaum@peds.bsd.uchicago.edu
2.Childrens Cancer Research Institute, St. Anna Kinderkrebsforschung, Kinderspitalgasse 6, A-1090 Vienna, Austria (IMA)(PFA); Center for Medical Genetics, University Hospital, De Pintelaan 185, B-9000 Gent, Belgium (FS)
Abstract
Neuroblastoma is a clinically heterogenous pediatric cancer of the sympathetic nervous system that originates from neural crest cells. It is the most common extracranial solid tumor in childhood and prognosis ranges from spontaneous tumor regression to aggressive disease resistant to multimodal therapy. Prognosis depends on patient characteristics and tumor biology that determine risk classification. Advancements in therapy reductions are merited for low- and intermediate-risk neuroblastoma patients, who generally have excellent outcomes. Of the patients with high-risk disease, only 50% achieve long-term survival, and therapeutic advancements are needed. Over the past several decades, genomic features such as germline mutations, somatic genetic aberrations, chromosome copy number, transcriptomics, and epigenetics have proven to contribute to the pathogenesis of neuroblastoma. The primary predisposition genes in familial neuroblastoma are ALK and PHOX2B. Sporadic neuroblastoma arises with complex pathogenesis, but chromosomal abnormalities and single-nucleotide polymorphisms have been identified to cooperatively lead to oncogenesis. These advances have led to new therapeutic approaches with the potential to improve outcomes for children with neuroblastoma.
Classification
Note
Classification
Clinics and Pathology
Embryonic origin
Etiology
Most neuroblastomas occur sporadically, with only 1-2% occurring in families in an autosomal dominant pattern (Matthay et al., 2016).
Epidemiology
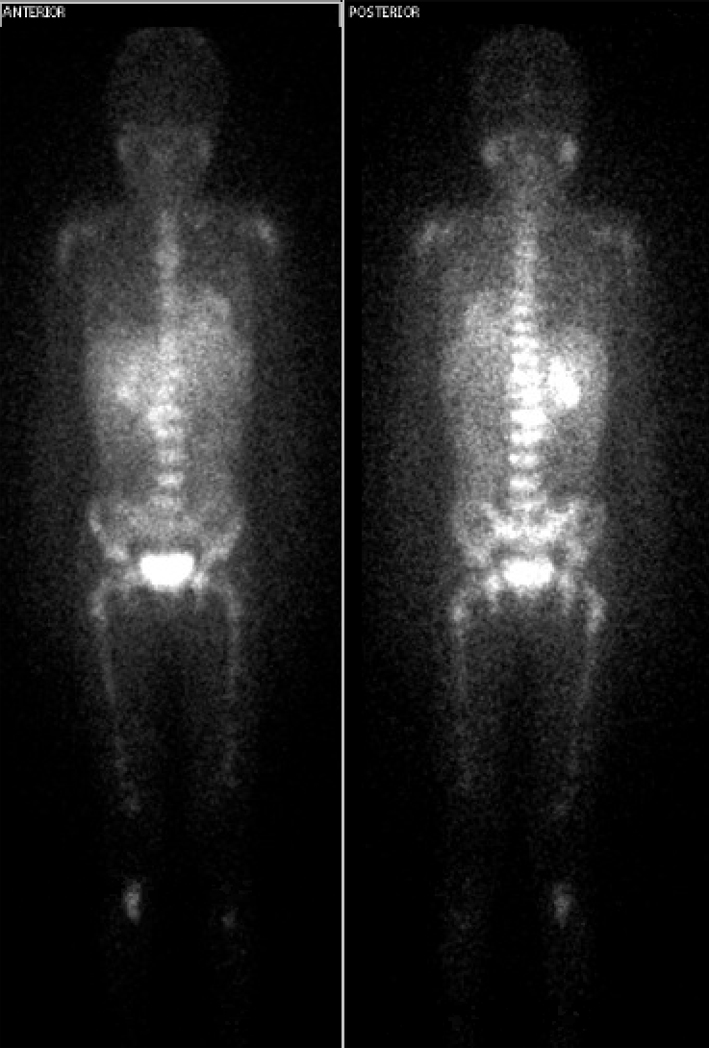
Pathology
- - Neuroblastoma (Schwannian stroma-poor)
- Ganglioneuroblastoma intermixed (Schwannian stroma-rich)
- Ganglioneuroma (Schwannian stroma-dominant), maturing subtype OR Ganglioneuroblastoma, well differentiated (Schwannian stroma-rich)
- Ganglioneuroblastoma nodular (composite).
Two of these categories are further divided into subgroups according to cellular differentiation signs, i.e. Neuroblastoma into: undifferentiated, poorly differentiated and differentiating; and Ganglioneuroblastoma into maturing and mature.
The degree of differentiation and stromal component of tumors can be used for prognostic assessment. Additionally, the age of the patient at diagnosis (below 1.5, between 1.5 and 5 years or over 5 years) is included as well as the number of mitotic and karyorrhectic (apoptotic) cells in the category of Neuroblastoma and the nodular part of Ganglioneuroma nodular.
Favorable features include:
Unfavorable features include:
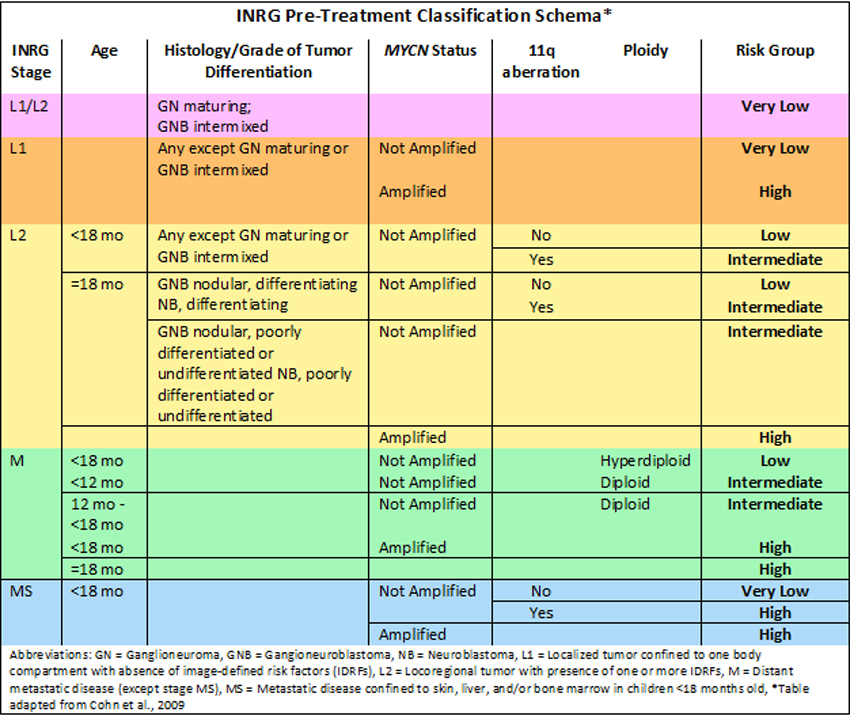
Prognostic Factors: As neuroblastoma is a heterogeneous disease, risk stratification according to clinical features of the patient and biologic features of the tumor has allowed for an accurate assessment of prognosis and risk-adapted therapy. To facilitate clinical research and improve the outcome of children with neuroblastoma, investigators from the major cooperative groups developed a consensus pre-treatment International Neuroblastoma Risk Group (INRG) classification system. The task force collected data on 36 prognostic variables on 8,800 children enrolled on cooperative group studies and identified the seven factors that were highly statistically significant and also considered clinically relevant (Cohn et al., 2009).
- Tumor Stage:
- -Stage L1: Locoregional tumor not involving vital structures as defined by the image-defined risk factors (IDRF) and confined to one body part
- -Stage L2: Locoregional tumor with presence of one or more IDRF
- -Stage M: Distant metastatic disease (except Stage MS)
- -Stage MS: Metastatic disease confined to skin, liver, and/or bone marrow
- -Age at diagnosis - Age
Other features
Multiple somatically acquired genomic alterations that contribute to tumorigenesis and progression of disease have been described. Somatic changes in the tumor genome such as gene mutations, loss or gain of alleles, and variations in ploidy have been identified as important factors in in the development of neuroblastoma and contribute to its diverse phenotype.
Treatment
Neuroblastoma mass screening: Neuroblastoma mass screening was attempted in order to identify patients in a preclinical stage with the intention to decrease mortality. The results obtained by these efforts indicated that while the incidence of low-risk neuroblastoma increased dramatically, there was no decrease in the incidence or mortality of high-risk disease, suggesting that screening was ineffective and that low-risk and high-risk neuroblastoma are two biologically independent entities.
Evolution
Cytogenetics
Note
2p24 Amplification-(MYCN): The MYCN oncogene on chromosome 2p24 encodes a transcription factor that is known to cause malignant transformation. Amplification of the MYCN gene is noted in 20% of all primary neuroblastoma tumors. It is present in 50% of all high-risk tumors, and it is associated with aggressive disease and poor prognosis (Brodeur et al., 1984). MYCN amplification is used as a biomarker for risk stratification in the INRG classification system (Cohn et al., 2009).
17q gain: The gain of the distal portion of chromosome 17q is the most common copy number aberration in neuroblastoma. It is present in 50% of primary neuroblastomas, primarily those that are otherwise classified as high-risk (Bown et al., 1999).
1p LOH: LOH at chromosome 1p36 correlates with MYCN-amplification, metastatic disease, and older age. It is present in 23-35% of primary neuroblastoma tumors (Maris et al., 2000).
11q loss occurs in about 33% of neuroblastomas, primarily non-MYCN-amplified, high-risk tumors. Despite significant research efforts, no confirmed driver tumor suppressors have been identified on 1p or 11q (Attiyeh et al., 2005).
Genetics
Note
- PHOX2B: Loss-of-function mutations in paired-like homeobox 2B (PHOX2B) were the first discovered neuroblastoma predisposition genes. PHOX2B mutations are responsible for of 6-10% of familial neuroblastoma cases. These mutations also occur in approximately 2% of sporadic neuroblastoma cases (Trochet et al., 2004).
- ALK: In 2008, the anaplastic lymphoma kinase gene (ALK) was identified as the major familial neuroblastoma predisposition gene (Mossé et al., 2008). Three missense mutations in in ALK (R1192P, R1275Q, G1128A) were found to be present in most familial neuroblastoma cases identified. ALK mutations are also present in 10-12% of cases of sporadic neuroblastoma.
- KIF1B: KIF1B is a tumor suppressor gene that is likely involved in the development of neural crest tumors. Germline mutations in KIF1B have been found in patients who have developed neuronal tumors, including neuroblastoma and pheochromocytoma (Schlisio et al., 2008; Barr and Applebaum, 2018).
- Other cancer predisposition syndromes: While the mutations in PHOX2B, ALK, and KIF1B are specific for predisposition to developing neuroblastoma, there are several syndromes that can lead to the development of neuroblastoma including RAS pathway mutations ( Costello syndrome, Noonan syndrome, neurofibromatosis type I, and congenital central hypoventilation syndrome), Li-Fraumeni syndrome, ROHHAD syndrome (rapid-onset obesity with hypothalamic dysfunction, hypoventilation, autonomic dysregulation), Wiedemann-Beckwith syndrome, Weaver syndrome, and Familial Paraganglioma/Pheochromocytoma.
Sporadic (Nonfamilial) Neuroblastoma
Sporadic neuroblastoma is recognized to be a complex genetic disease. Large genome-wide association studies (GWAS) have identified common single nucleotide polymorphisms that influence neuroblastoma susceptibility and phenotype.
- BARD1 contains one of the most replicated signals. It has generally been thought of as a tumor suppressor, but in neuroblastoma, risk alleles are associated with increased expression of BARD1, which promotes growth (Capasso et al., 2009).
- LMO1 is a neuroblastoma oncogene and polymorphisms in the gene are associated with the development of high risk, clinically aggressive disease (Wang et al., 2011).
- LIN28B polymorphisms are strongly correlated to with predisposition to high-risk neuroblastoma. Variants in HACE1, TP53 have also been identified (Diskin et al., 2016).
- Variants in DUSP12 (1q23), HSD17B12 (11p11), DDX4 / IL31RA (5q11.2) are associated with low-risk neuroblastoma (Nguyen et al., 2011).
- Variants in CASC15, NBAT1 (6p22) influence susceptibility to high-risk neuroblastoma (Russell et al., 2015).
- Polymorphisms in KIF15 are associated with the development of MYCN amplified neuroblastoma (Hungate et al., 2017) and those in MMP20 are associated with somatic 11q deletion (Chang et al., 2017).
Genes Involved and Proteins
Gene name
Location
Note
Protein description
Germinal mutations
Somatic mutations
Note
ATRX: Loss-of-function mutations or deletions in the RNA helicase alpha-thalassemia/mental retardation syndrome X-linked (ATRX) have been observed in neuroblastoma (10% of cases) and are often found in older patients (age at diagnosis >5). ATRX plays a role in chromatin remodeling, nucleosome assembly, and telomere maintenance. ATRX mutations are mutually exclusive with MYCN-amplification and TERT mutations (Pugh et al., 2013).
TERT: Genomic rearrangements in telomerase reverse transcriptase (TERT), a target of MYCN, can lead to neuroblastoma. It has been proposed that all neuroblastomas need a pathway to activate TERT or bypass the pathway via ATRX mutation (Valentijn et al., 2015).
Gene name
Location
Note
Protein description
Somatic mutations
Gene name
Location
Note
Protein description
Somatic mutations
Gene name
Location
Note
Protein description
Somatic mutations
To be Noted
Note
Article Bibliography
Citation
Caileigh Pudela ; Skye Balyasny ; Mark A. Applebaum
Nervous system: Embryonal tumors: Neuroblastoma
Atlas Genet Cytogenet Oncol Haematol. 2019-10-01
Online version: http://atlasgeneticsoncology.org/solid-tumor/5002/nervous-system-embryonal-tumors-neuroblastoma
Historical Card
2007-12-01 Nervous system: Embryonal tumors: Neuroblastoma by Inge M Ambros,Frank Speleman,Peter F Ambros Affiliation
2001-09-01 Nervous system: Embryonal tumors: Neuroblastoma by Yasuhiko Kaneko Affiliation
1998-02-01 Nervous system: Embryonal tumors: Neuroblastoma by Jérôme Couturier,Daniel Satgé Affiliation
