Identity
HGNC
LOCATION
17q25.1
LOCUSID
ALIAS
ASH,EGFRBP-GRB2,Grb3-3,MST084,MSTP084,NCKAP2
FUSION GENES
DNA/RNA
Transcription
The GRB2 gene structure consists of five exons (ranging from 78 to 186 bp) and four introns (from approximately 1 to approximately 7 kb). Two human mRNA transcript variants arise from alternative splicing. GRB2 variant 1 mRNA encodes protein isoform 1, which is longer. Variant 2 mRNA, which encodes protein isoform 2, lacks an in-frame exon present in the 3 coding region of variant 1 encompassing residues 59 - 100 of the mature protein (see Protein, below).
Pseudogene
At least one potential human pseudogene may exist: LOC391157. A pseudogene of the mouse Grb2 homolog, known as Grb2-ps1 (growth factor receptor bound protein 2, pseudogene 1) also has been identified.
Proteins
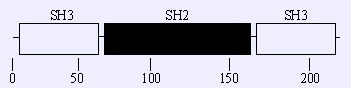
A schematic representation of the domain structure of GRB2, which consists of a single Src homology 2 (SH2) domain (residues 59 - 152) flanked by two SH3 domains (amino-terminal: residues 3 - 54; carboxy-terminal: residues 160-212).
Description
GRB2 (isoform 1) is a 217 residue protein with an expected molecular mass of 25,206 Da. GRB2 protein has homology to non-catalytic regions of c-Src, consisting of a single Src homology 2 (SH2) domain flanked by two Src homology 3 (SH3) domains. GRB2 isoform 2, encoded by an alternatively spliced mRNA transcript known as variant 2, has a deletion in the amino-terminal portion of the SH2 domain encompassing residues 59 - 100 of isoform 1. This protein isoform, known originally as GRB3-3, does not bind to phosphotyrosyl-containing proteins like isoform 1, but retains two functional SH3 domains.
Expression
Expressed in virtually all embryonic and adult tissues.
Localisation
Primarily cytosolic, but transient plasma membrane and nuclear localizations have been reported.
Function
Cell surface receptor signaling - The two GRB2 SH3 domains bind to the proline-rich regions of the guanine nucleotide releasing factor son of sevenless (SOS-1) protein, and the GRB2-SOS-1 complex preexists in the cytoplasm of resting cells. Phosphotyrosyl residues on in the context of the motif NH2- pYXNX-COOH (where pY represents phosphotyrosine, N represents asparagine, and X represents any other residue) are selectively recognized by the GRB2 SH2 domain. Growth factor receptor tyrosine kinases (RTKs), including those for epidermal growth factor (EGF), fibroblast growth factor, nerve growth factor (TrkA/TrkB), platelet-derived growth factor, colony-stimulating factor-1, and hepatocyte growth factor (HGF), as well as non-receptor tyrosine kinases (TKs) such as BCR-Abl and focal adhesion kinase (FAK), intracellular effectors such as insulin receptor substrate-1 and Shc, and phosphotyrosine phosphatases such as SHP-2 (PTPN11) and receptor-like tyrosine phosphatase alpha, all conditionally possess the pYXNX motif. Note that the environmental cue leading to protein tyrosyl phosphorylation on an appropriate GRB2 recognition motif is independent of GRB2 interaction; thus, ligand independent EGFR activation, such as growth hormone-induced EGFR tyrosine phosphorylation by JAK2, also leads to GRB2-mediated ERK kinase pathway activation and c-fos expression. Similarly, mechanical stress leading to increased angiotensin II production and transactivation of EGFR and other intracellular kinases implicates GRB2 recruitment in cardiac hypertrophy and myocardial remodeling.
In many mitogenic signaling pathways, recruitment of GRB2 from the cytosol, where it is already bound to the guanine nucleotide exchange factor SOS-1 via its amino-terminal SH3 domain, brings SOS1 in close proximity to Ras at the plasma membrane. Ras, a small GTPase in the GDP-bound inactive state in quiescent cells, then undergoes nucleotide exchange of GDP for GTP, which facilitates binding of the serine/threonine protein kinase Raf-1 and its subsequent activation. This initiates a cascade of kinase activation: activated Raf-1 phosphorylates and activates MEK1/MEK2, which in turn phosphorylate and stimulate the MAP kinases ERK1/ERK2. Activated ERKs translocate to the nucleus and phosphorylate transcription factors such as Elk-1, STAT1, STAT3 and Myc, activating gene expression. In parallel, the phosphatidyl inositol 3-kinase (PI3K)/Akt pathway is activated via the adaptor Gab1, which is bound to the GRB2 carboxyl-terminal SH3 domain in many epithelial cell types. The gene expression programs activated by these pathways initiate a spectrum of fundamental cellular activities including proliferation, growth (increase in cell size), differentiation and survival. These processes are critical for normal embryonic development and adult homeostasis, and are frequently aberrantly activated in cancer.
Stimulation of the T cell antigen receptor (TCR) induces the tyrosine phosphorylation of a variety of cellular proteins, including a protein called p36-38 or Linker for Activation of T cells (LAT), a protein tightly associated with the plasma membrane. Tyrosyl-phosphorylated sequences of LAT bind to the GRB2 SH2 domain. In these cells the SH3 domains of GRB2 bind Vav-family proteins, guanine nucleotide exchange factors for Rho-family GTPases. These interactions are essential for TCR-induced calcium flux and activation of the MAP kinase cascade, ultimately leading to T cell proliferation and effector functions.
Receptor endocytosis and ubiquitinylation - Upon ligand-dependant activation of EGFR TK, c-Cbl binds to the EGFR directly through its SH2 domain and indirectly through its SH3 domain. c-Cbl binding and its consequential phosphorylation results in activation of the E3 ubiquitin ligase complex of which c-Cbl is a component, resulting in receptor ubiquitinylation. GRB2 also regulates internalization of EGF receptors through clathrin-coated pits.
Actin-based cell motility - GRB2 participates directly in the regulation of actin filament formation and actin-based cell motility. GRB2 is a critical link between Wiskott-Aldrich Syndrome protein (WASp) and the actin cytoskeleton; WAS patients show defects in T cell polarization and migration in response to physiologic stimuli, resulting in thrombocytopenia, eczema and immunodeficiency. Studies of WASp function and the intracellular motility of invasive microbial pathogens such as Listeria monocytogenes and Vaccinia virus helped to elucidate an important role for GRB2 in directly promoting actin based motility. In most mammalian cells, the WASp family member N-WASp interacts with the Arp2/3 complex and G-actin to stimulate actin polymerization. N-WASp activity is enhanced by other effectors such as Nck, Cdc42 and GRB2; disruption of GRB2 SH3 or SH2 domains diminishes actin polymerization and thus actin-based motility.
In many mitogenic signaling pathways, recruitment of GRB2 from the cytosol, where it is already bound to the guanine nucleotide exchange factor SOS-1 via its amino-terminal SH3 domain, brings SOS1 in close proximity to Ras at the plasma membrane. Ras, a small GTPase in the GDP-bound inactive state in quiescent cells, then undergoes nucleotide exchange of GDP for GTP, which facilitates binding of the serine/threonine protein kinase Raf-1 and its subsequent activation. This initiates a cascade of kinase activation: activated Raf-1 phosphorylates and activates MEK1/MEK2, which in turn phosphorylate and stimulate the MAP kinases ERK1/ERK2. Activated ERKs translocate to the nucleus and phosphorylate transcription factors such as Elk-1, STAT1, STAT3 and Myc, activating gene expression. In parallel, the phosphatidyl inositol 3-kinase (PI3K)/Akt pathway is activated via the adaptor Gab1, which is bound to the GRB2 carboxyl-terminal SH3 domain in many epithelial cell types. The gene expression programs activated by these pathways initiate a spectrum of fundamental cellular activities including proliferation, growth (increase in cell size), differentiation and survival. These processes are critical for normal embryonic development and adult homeostasis, and are frequently aberrantly activated in cancer.
Stimulation of the T cell antigen receptor (TCR) induces the tyrosine phosphorylation of a variety of cellular proteins, including a protein called p36-38 or Linker for Activation of T cells (LAT), a protein tightly associated with the plasma membrane. Tyrosyl-phosphorylated sequences of LAT bind to the GRB2 SH2 domain. In these cells the SH3 domains of GRB2 bind Vav-family proteins, guanine nucleotide exchange factors for Rho-family GTPases. These interactions are essential for TCR-induced calcium flux and activation of the MAP kinase cascade, ultimately leading to T cell proliferation and effector functions.
Receptor endocytosis and ubiquitinylation - Upon ligand-dependant activation of EGFR TK, c-Cbl binds to the EGFR directly through its SH2 domain and indirectly through its SH3 domain. c-Cbl binding and its consequential phosphorylation results in activation of the E3 ubiquitin ligase complex of which c-Cbl is a component, resulting in receptor ubiquitinylation. GRB2 also regulates internalization of EGF receptors through clathrin-coated pits.
Actin-based cell motility - GRB2 participates directly in the regulation of actin filament formation and actin-based cell motility. GRB2 is a critical link between Wiskott-Aldrich Syndrome protein (WASp) and the actin cytoskeleton; WAS patients show defects in T cell polarization and migration in response to physiologic stimuli, resulting in thrombocytopenia, eczema and immunodeficiency. Studies of WASp function and the intracellular motility of invasive microbial pathogens such as Listeria monocytogenes and Vaccinia virus helped to elucidate an important role for GRB2 in directly promoting actin based motility. In most mammalian cells, the WASp family member N-WASp interacts with the Arp2/3 complex and G-actin to stimulate actin polymerization. N-WASp activity is enhanced by other effectors such as Nck, Cdc42 and GRB2; disruption of GRB2 SH3 or SH2 domains diminishes actin polymerization and thus actin-based motility.
Homology
GRB2 amino acid sequence is very highly conserved among species. Human GRB2 shows 50% overall amino acid sequence identity with S. cerevisiae YPR154w, 58% identity with Sem-5 of C. elegans, 66% identity with the D. melanogaster homolog Drk and over 99% identity with both rat and mouse homologs.
Mutations
Note
No known naturally-occurring mutations in human GRB2 have been reported.
Implicated in
Entity name
Normal embryogenesis
Note
A null mutation introduced into the mouse gene for Grb2 was used to demonstrate that Grb2 is required during embryogenesis for the differentiation of endodermal cells and epiblast formation. Replacing the carboxy-terminus of SOS-1 with the Grb2 SH2 domain yielded a fusion protein that rescued the defects caused by this Grb2 mutation. Grb2 signaling primarily regulates differentiation, rather than proliferation, in the early mouse embryo.
Entity name
Cardiac hypertrophy
Note
Engineered Grb2 +/- mice subjected to cardiac stress failed to activate p38 MAP kinase (MAPK14) and Jun N-terminal kinase (JNK), and the cardiac hypertrophy and fibrosis observed in normal mice were blocked. Transgenic mice with dominant-negative forms of MAPK p38-alpha and p38-beta developed cardiac hypertrophy but were resistant to cardiac fibrosis when subjected to cardiac stress. These and other findings suggest that Grb2 activity is essential for cardiac hypertrophy and fibrosis in response to pressure overload, and that different signaling pathways downstream of Grb2 regulate fibrosis, fetal gene induction, and cardiomyocyte growth.
Entity name
Cancer
Note
As a pivotal activator of cell-cycle control and motility pathways downstream of several growth factor receptors, GRB2 is involved in oncogenic signaling in a wide variety of human tumors. For example, GRB2 directly interacts with SOS-1 and the Bcr portion of the Bcr-Abl fusion protein, a tyrosine kinase oncoprotein which has been implicated in the pathogenesis of Philadelphia chromosome positive leukemias, such as CML, ALL, and AML. GRB2 is rate limiting for mammary carcinomas induced by polyomavirus middle T antigen. GRB2 over expression has been reported in human breast, bladder and prostate cancer cell lines. Selective small molecule inhibitors of GRB2 SH2 domain binding block solid tumor metastasis in animal models.
Article Bibliography
Other Information
Locus ID:
NCBI: 2885
MIM: 108355
HGNC: 4566
Ensembl: ENSG00000177885
Variants:
dbSNP: 2885
ClinVar: 2885
TCGA: ENSG00000177885
COSMIC: GRB2
RNA/Proteins
Expression (GTEx)
Pathways
Protein levels (Protein atlas)
PharmGKB
References
Citation
Gagani Athauda ; Donald P Bottaro
GRB2 (Growth factor receptor-bound protein 2)
Atlas Genet Cytogenet Oncol Haematol. 2007-05-01
Online version: http://atlasgeneticsoncology.org/gene/386/grb2-(growth-factor-receptor-bound-protein-2)
