GATA3 (GATA binding protein 3)
2011-12-01 Mathieu Tremblay , Maxime Bouchard AffiliationGoodman Cancer Research Centre, Department of Biochemistry, McGill University, Montreal, Canada
DNA/RNA
Description
Transcription
Proteins
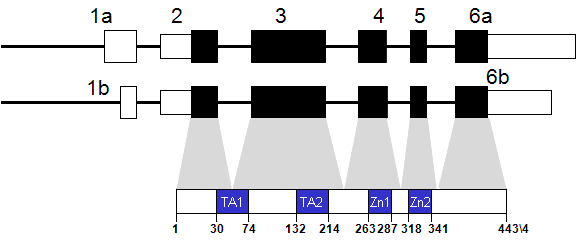
Description
Expression
Localisation
Function
Gata3 gene inactivation in the mouse is embryonic lethal at mid-gestation (between embryonic days E11 and E12) (Tsai et al., 1994; Pandolfi et al., 1995). These mice display massive internal bleeding, marked growth retardation, severe deformities of the brain and spinal cord, and gross aberrations in fetal liver hematopoiesis. Lethality of Gata3 mutant embryos can be rescued by administration of catechol intermediates during pregnancy as it corrects the reduction in noradrenalin synthesis in the sympathetic nervous system (SNS) caused by reduced expression of tyrosine hydroxylase (TH) and dopamine beta-hydroxylase (DBH). Pharmacologically rescued mutant embryos present developmental defects in structures derived from cephalic neural crest cells (Lim et al., 2000).
In the kidney, Gata3 is important for nephric (Wolffian) duct elongation and metanephric kidney induction (Grote et al., 2006; Grote et al., 2008). Conditional inactivation of Gata3 in the nephric duct leads to hydronephrosis and defective ureter maturation, partly due to the downregulation of the receptor tyrosine kinase gene Ret (Song et al., 2009; Chia et al., 2011).
Gata3 plays an important role in mammary gland maturation and cancer. The conditional deletion of Gata3 in the mouse mammary epithelium is associated with a failure in terminal end bud formation at puberty causing severe defects in mammary development. Moreover, Gata3 loss in adult mice leads to an expansion of undifferentiated luminal cells and basement-membrane detachment, which promotes tumor dissemination (Kouros-Mehr et al., 2006; Asselin-Labat et al., 2007; Kouros-Mehr et al., 2008; Kouros-Mehr et al., 2008; Dydensborg et al., 2009).
Reexpression of Gata3 drives invasive breast cancer cells to undergo the reversal of epithelial-mesenchymal transition, reducing both the tumorigenicity and metastatic potential through reduction of lysyl oxidase (LOX) expression, a metastasis-promoting, matrix-remodeling protein (Chu et al., 2011; Yan et al., 2010). Moreover, Gata3 interact with BRCA1 to repress the expression of genes associated with triple-negative and basal-like breast cancer (BLBCs) including Foxc1, Foxc2, Cxcl1 and P-cadherin. Loss of GATA3 expression also contributes to drug resistance and epithelial-to-mesenchymal transition-like phenotypes associated with aggressive BLBCs (Tkocz et al., 2011).
In T cells, Gata3 acts at multiple stages of thymocyte differentiation. It is indispensable for early thymic progenitor differentiation (Hosoya et al., 2009) and for thymocytes to pass through beta selection and T cell commitment. Gata3 is also necessary for single-positive CD4 thymocyte development as well as for Th1-Th2 lineage commitment (Ting et al., 1996; Zhang et al., 1997; Zheng and Flavell, 1997; Zhang et al., 1998; Pai et al., 2003). As master regulator of Th2 lineage commitment, GATA3 acts either as a transcriptional activator or repressor through direct action at many critical loci encoding cytokines, cytokine receptors, signaling molecules as well as transcription factors that are involved in the regulation of T(h)1 and T(h)2 differentiation (Jenner et al., 2009). For example, it regulates the expression of Th2 lineage specific cytokine gene such as IL5 and repress the Th1 lineage specific genes IL-12 receptor β2 and STAT4 as well as neutralizing RUNX3 function through protein-protein interaction. Mice lacking Gata3 produce IFN-gamma rather than Th2 cytokines (IL5 and IL13) in response to infection (Zhu et al., 2004). It acts in mutual opposition to the transcription factor T-bet, as T-bet promotes whereas GATA3 represses Fut7 transcription (Hwang et al., 2005). It also acts with Tbx21 to regulate cell lineage-specific expression of lymphocyte homing receptors and cytokine in both Th1 and Th2 lymphocyte subsets (Chen et al., 2006). Enforced expression of Gata3 during T cell development induced CD4(+)CD8(+) double-positive (DP) T cell lymphoma (Nawijn et al., 2001a; Nawijn et al., 2001b).
Gata3 is essential for the expression of the cytokines IL-4, IL-5 and IL-13 that mediate allergic inflammation. Gata3 overexpression causes enhanced allergen-induced airway inflammation and airway remodeling, including subepithelial fibrosis, and smooth muscle cell hyperplasia (Kiwamoto et al., 2006). It additionally has a critical function in regulatory T cells and immune tolerance since deletion of Gata3 specifically in regulatory T cells led to a spontaneous inflammatory disorder in mice (Wang et al., 2011).
Gata3 is critical for the differentiation and survival of parathyroid progenitor cells through regulation of GCM2/B (Grigorieva et al., 2010). Gata3 is essential for the survival but not the differentiation of sympathetic neurons and adrenal chromaffin cells (Tsarovina et al., 2010) and acts with Hand2 to induce noradrenergic genes during development (Pellegrino et al., 2011).
Gata3 drives trophoblast differentiation and has been shown to induce a trophoblast cell fate in embryonic stem cells (Ralston et al., 2010). Gata3 and its close paralog Gata2 are important for trophectoderm lineage specification (Ray et al., 2009).
During adipogenesis, Gata3 is a negative regulator of differentiation which needs to be downregulated to permit expression of the peroxisome proliferator-activated receptor-gamma and preadipocyte to adipocyte transition (Tong et al., 2000).
In keratinocytes, Gata3 is a key regulator of KLK1 expression and is involved in growth control and the maintenance of a differentiated state in epithelial cells (Son do et al., 2009).
In hair follicle morphogenesis Gata3 controls cell fate decision between the inner root sheath and hair shaft cell (Kaufman et al., 2003; Kurek et al., 2007).
Gata3 is essential for lens cells differentiation and proper cell cycle control (Maeda et al., 2009) as well as in the morphogenesis of the mouse inner ear (Karis et al., 2001; Lilleväli et al., 2004).
It plays an essential role during angiogenesis through ANGPT1-TEK and Ang-1-Tie2-mediated signaling in large vessel endothelial cells.
A role for Gata3 in the developing heart was revealed by pharmacological rescue of Gata3-null embryos, which survive until birth and harbor ventricular septal defect (VSD), double-outlet of right ventricle (DORV), anomalies of the aortic arch (AAA) and persistent truncus arteriosus (PTA) (Raid et al., 2009).
Homology
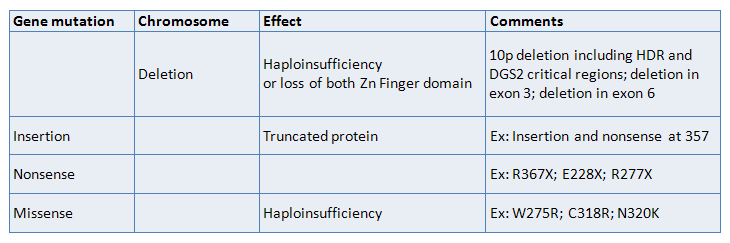
Mutations
Germinal
Somatic
Implicated in
GATA3 is an important predictor of disease outcome in breast cancer patients whereby low GATA3 expression was a significant predictor of disease-related death (Yoon et al., 2010).
- Hypoparathyroidism.
- Sensorineural deafness, bilateral, symmetric, deficit affecting all frequencies but slightly more marked at the higher end of the frequency range.
- Renal defects such as aplasia, dysplasia and vesicoureteral reflux, associated or not to genital tract malformation.
- GATA3 gene mutations leading to functional haploinsufficiency.
Article Bibliography
Other Information
Locus ID:
NCBI: 2625
MIM: 131320
HGNC: 4172
Ensembl: ENSG00000107485
Variants:
dbSNP: 2625
ClinVar: 2625
TCGA: ENSG00000107485
COSMIC: GATA3
RNA/Proteins
Expression (GTEx)
Pathways
Protein levels (Protein atlas)
PharmGKB
References
Citation
Mathieu Tremblay ; Maxime Bouchard
GATA3 (GATA binding protein 3)
Atlas Genet Cytogenet Oncol Haematol. 2011-12-01
Online version: http://atlasgeneticsoncology.org/gene/107/gata3id107ch10p14
